According to the CDC , that’s almost double the number of deaths from Covid19 in the same year. Breast, prostate, lung and colorectal cancers top the charts for both incidence and cancer death rates in the US, but in a study published in February 2021, that landscape is set to change notably by 2040.
In 2020, there were an estimated 1.8 million diagnoses and more than 600,000 deaths from cancer in the US.
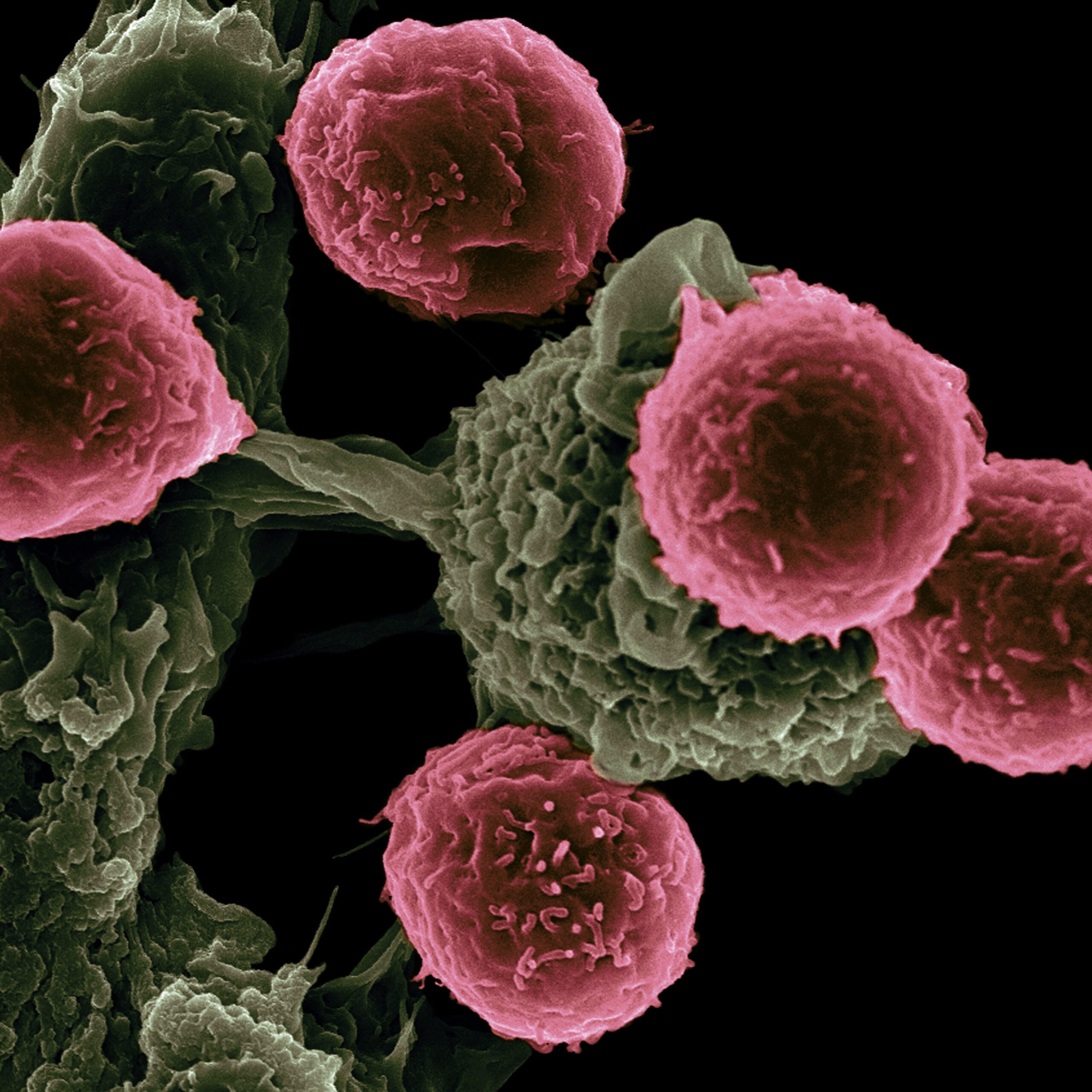
But forecasting that puts CCA as the third leading cause of cancer death within 20 years demonstrates just how misleading the term ‘rare’ can be. With a median survival rate of less than a year, it has never been more important to invest in the research and clinical trials needed to bring life-saving drugs to market for those facing CCA. If we wait until people need it, it will already be too late.
The discovery of common gene mutations in certain cancers has the potential to be a gamechanger when it comes to drug development – not only for people fighting CCA, but as a step towards the future of cancer treatment as a whole. Common genomic mutations, such as IDH1, FGFR2 and PIK3CA, have been found in patients with breast, ovarian, colon, lung, pancreatic and brain cancer, so a step towards success for cholangiocarcinoma brings hope to many many more.
We couldn’t think of a more worthy cause to put our money behind, which is why we are backing the pioneering research of Dr Lipika Goyal, Dr Dejan Juric and their expert teams at Massachusetts General Hospital (MGH).

Dr Dr Dejan Juric MD is a renowned expert in personalized cancer medicine and leads the Henri & Belinda Termeer Cancer Center at MGH. He is heavily involved in early drug development of targeted cancer therapeutics, and having helped develop the first gene-targeted treatment for women with HR+ breast cancer with the PIK3CA gene mutation, he is now investigating whether PIK3CA is a valid target for other cancers, such as clear cell carcinoma of the ovary.
Dr. Lipika Goyal MD, MPhil is a recognized expert in IDH1 and FGFR2 mutant cholangiocarcinoma and is leading research into tumor heterogeneity and drug resistance in CCA. She has been instrumental in the development of drugs that are slated to gain FDA approval for these cancers in 2021, such as futinatinib which has recently been granted ‘Breakthrough Therapy Designation’ for advanced CCA . This means it will get expedited review – hugely encouraging for patients with CCA with limited options and little time.
Breakthroughs like this are proof of what funding into targeted research and clinical trials can achieve – and is why we do what we do. Targeted therapy options beyond palliative chemotherapy gives people hope; they could be the difference between life and death for people facing the worst odds. But saving lives costs money.
It typically costs between $25,000 and $35,000 to enrol one patient into a Phase 1 clinical trial. As a result, it costs a minimum of $1M to launch and complete an end-to-end Phase 1 study of 40 patients. That’s without the practical costs for patients getting access to these trials. We are committed to funding as many Phase 1 and 2 clinical trials as we can, to produce robust data on the path to FDA approval and to bring hope backed by scientific rationale to those who need it most.
In the six months since The Rare Initiative launched, we have raised over $425,000. That’s enough to enrol around 15 people into clinical trials, and we’re only just getting started. This is a moment of opportunity; a game-changing shift in the development of life-saving drugs that are predicted to be increasingly necessary, and that could fundamentally change the way cancer is treated.
Our ambition is clear, but we need your support to get us there. To be part of the solution and to help us on our way to FDA approval, please consider making a donation.
Learn more about the research we are funding, join our fundraising movement and follow our tribe on Instagram and Facebook.

